Embark on a scientific expedition with the Naming of Ionic Compounds Worksheet, a comprehensive guide that empowers you to master the intricacies of naming these essential chemical entities. Dive into the fascinating world of ions, where positive and negative charges dance in harmonious union, forming the building blocks of countless compounds that shape our universe.
Through a systematic approach, this worksheet unveils the fundamental principles of ionic compound nomenclature, equipping you with the knowledge to decipher their chemical identities with precision. Prepare to unravel the secrets of ionic compounds and embark on a journey of scientific discovery.
Definitions
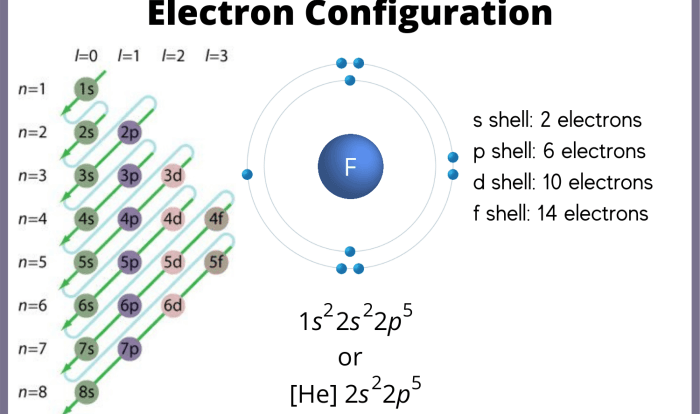
Ionic compounds are formed when a metal loses one or more electrons to a non-metal. The metal becomes a positively charged ion, called a cation, and the non-metal becomes a negatively charged ion, called an anion. The oppositely charged ions are attracted to each other by electrostatic forces, forming an ionic bond.
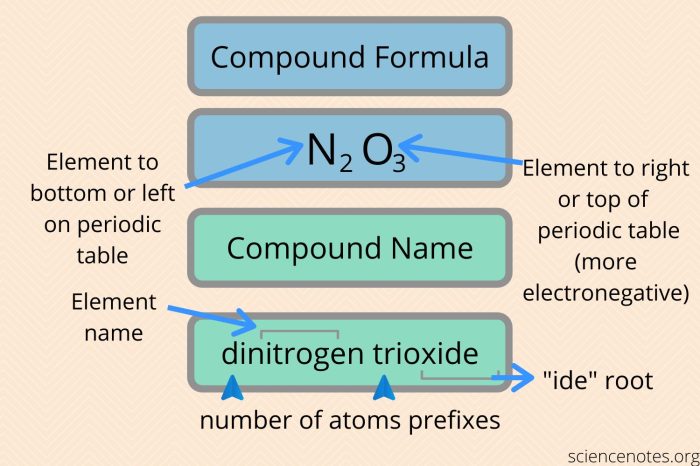
The name of an ionic compound is based on the names of the cation and anion. The cation is always named first, followed by the anion. The name of the cation is the same as the name of the metal.
The name of the anion is the root of the non-metal’s name, followed by the suffix -ide.
Exceptions
There are a few exceptions to the rules for naming ionic compounds. For example, the cation of iron(II) is named ferrous, and the cation of iron(III) is named ferric. The anion of oxygen is named oxide, and the anion of hydrogen is named hydride.
Naming Rules
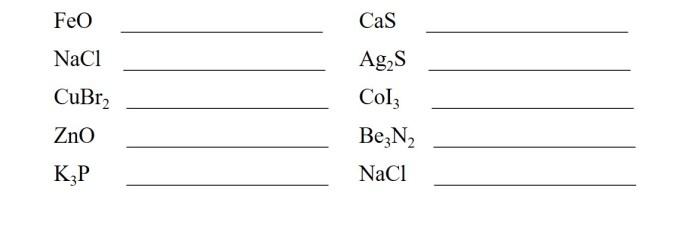
Ionic compounds are named based on the names of the ions that compose them. There are general rules for naming both cations and anions.
The name of a cation is the same as the name of the element from which it is formed. For example, the cation formed from sodium is called sodium ion.
The name of an anion is formed by adding the suffix “-ide” to the root of the name of the element from which it is formed. For example, the anion formed from chlorine is called chloride ion.
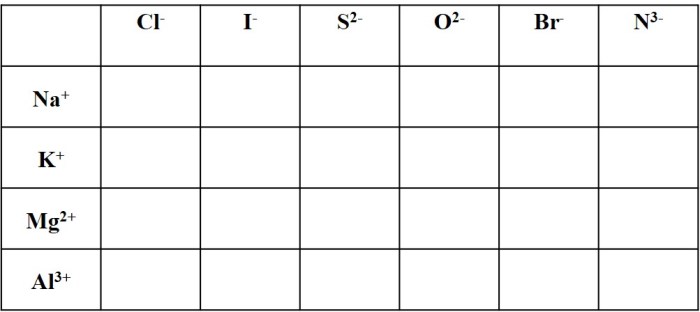
Naming Cations
When naming cations, the following rules apply:
- For cations of metals that form only one type of cation, the name of the cation is the same as the name of the metal. For example, the cation of sodium is called sodium ion.
- For cations of metals that form more than one type of cation, the name of the cation includes the Roman numeral of the charge of the cation. For example, the cation of iron(II) is called iron(II) ion, and the cation of iron(III) is called iron(III) ion.
Naming Anions
When naming anions, the following rules apply:
- For anions of nonmetals that form only one type of anion, the name of the anion is formed by adding the suffix “-ide” to the root of the name of the nonmetal. For example, the anion of chlorine is called chloride ion.
- For anions of nonmetals that form more than one type of anion, the name of the anion includes the suffix “-ide” and the Roman numeral of the charge of the anion. For example, the anion of sulfur(II) is called sulfide ion, and the anion of sulfur(VI) is called sulfate ion.
Practice Problems

To enhance your understanding of ionic compound naming, let’s engage in a series of practice problems.
The following table provides a structured approach to practice naming ionic compounds, including the chemical formula, cation name, anion name, and the corresponding ionic compound name.
Table of Practice Problems, Naming of ionic compounds worksheet
| Chemical Formula | Cation Name | Anion Name | Ionic Compound Name |
|---|---|---|---|
| NaCl | Sodium | Chloride | Sodium chloride |
| CaO | Calcium | Oxide | Calcium oxide |
| Al2O3 | Aluminum | Oxide | Aluminum oxide |
| NH4Cl | Ammonium | Chloride | Ammonium chloride |
| Fe2O3 | Iron(III) | Oxide | Iron(III) oxide |
4. Examples and Exceptions: Naming Of Ionic Compounds Worksheet
Examples of Correctly Named Ionic Compounds
Correctly named ionic compounds adhere to the naming rules discussed earlier. Here are some examples:
- NaCl: Sodium chloride
- MgO: Magnesium oxide
- CaF 2: Calcium fluoride
- Al 2O 3: Aluminum oxide
- Fe 2O 3: Iron(III) oxide
Exceptions to the Naming Rules
While the naming rules provide a general framework, there are some exceptions to consider:
- Ammonium (NH4+): The ammonium ion is named as if it were a metal, despite being a polyatomic ion. In compounds, it is treated as a monovalent cation and named “ammonium.”
- Mercury(II):The Roman numeral (II) is used to indicate the +2 oxidation state of mercury in compounds, even though it is not a variable-charge metal.
- Iron(II) and Iron(III):Iron can exhibit two common oxidation states (+2 and +3). These are denoted using Roman numerals in the compound name, as in “iron(II) oxide” (FeO) and “iron(III) oxide” (Fe 2O 3).
- Compounds with Polyatomic Ions:When an ionic compound contains a polyatomic ion, the name of the ion is used as part of the compound name. For example, “sodium sulfate” (Na 2SO 4) contains the polyatomic ion sulfate (SO 42-).
Resources
To further your understanding of naming ionic compounds, we highly recommend exploring the following resources:
Online Resources:
- Khan Academy: Naming Ionic Compounds
- ThoughtCo: How to Name Ionic Compounds
- Michigan State University: Nomenclature of Ionic Compounds
Textbooks:
- Chemistry: The Central Scienceby Theodore L. Brown, H. Eugene LeMay, Jr., Bruce E. Bursten, and Catherine J.
Murphy
- Chemistryby Raymond Chang and Kenneth A. Goldsby
- Inorganic Chemistryby C. E. Housecroft and A. G. Sharpe
Additional Practice Exercises:
To solidify your understanding, we encourage you to complete the following practice exercises:
- Khan Academy: Naming Ionic Compounds Practice
- Michigan State University: Nomenclature of Ionic Compounds Exercises
- Education.com: Naming Ionic Compounds Practice
FAQ Section
What is the purpose of this worksheet?
This worksheet is designed to provide a comprehensive guide to naming ionic compounds, empowering you to master the fundamental principles of ionic compound nomenclature.
What topics are covered in this worksheet?
This worksheet covers the definitions of ionic compounds, the general rules for naming ionic compounds, specific rules for naming cations and anions, practice problems for naming ionic compounds, and examples and exceptions to the naming rules.
How can I use this worksheet?
This worksheet can be used as a self-study guide, a classroom resource, or a supplement to your existing chemistry curriculum. It is designed to be accessible and engaging for students of all levels.