Draw the electron configuration for a neutral atom of manganese. – In the realm of atomic chemistry, electron configuration plays a pivotal role in unraveling the fundamental structure and properties of elements. This article delves into the intricate details of drawing the electron configuration for a neutral atom of manganese, providing a comprehensive guide for students and researchers alike.
Manganese, an essential transition metal, exhibits a unique electron configuration that governs its chemical behavior and reactivity. By understanding the principles of electron configuration, we can gain valuable insights into the nature of this fascinating element.
Introduction to Electron Configuration
Electron configuration refers to the distribution of electrons in the orbitals around the nucleus of an atom. It plays a crucial role in determining the chemical properties and behavior of elements.
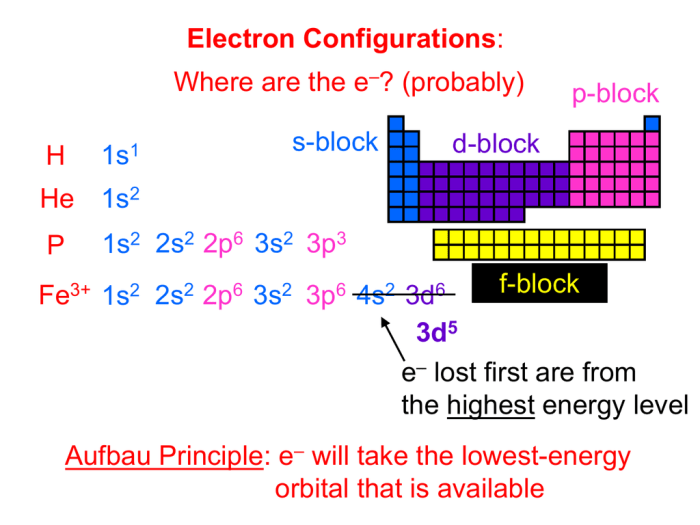
The Aufbau principle states that electrons fill orbitals in order of increasing energy, while Hund’s rule specifies that orbitals of equal energy are filled with unpaired electrons before pairing occurs.
Atomic Number and Electron Configuration
The atomic number of an element represents the number of protons in its nucleus, which is equal to the number of electrons in a neutral atom.
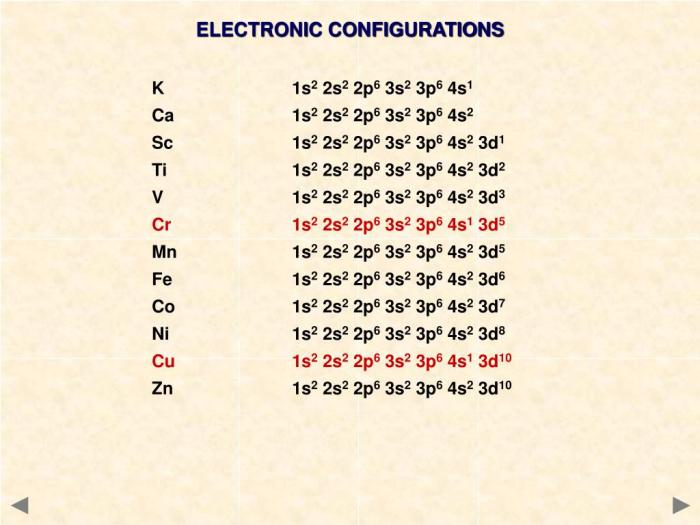
Manganese (Mn) has an atomic number of 25, indicating that a neutral manganese atom contains 25 electrons.
Orbital Arrangement, Draw the electron configuration for a neutral atom of manganese.
Electrons occupy specific energy levels, known as shells, which are further divided into subshells or orbitals.
- s orbitals:Spherical
- p orbitals:Dumbbell-shaped, with three orientations (px, py, pz)
- d orbitals:Complex shapes, with five orientations (dxy, dyz, dxz, dx2-y2, dz2)
- f orbitals:Very complex shapes, with seven orientations
Electron Distribution in Manganese
To determine the electron configuration of manganese, we fill the orbitals with electrons in the order of increasing energy:
- 1s2
- 2s 22p 6
- 3s 23p 63d 5
- 4s 2
The orbital diagram below represents this configuration:
1s 2s 2p 3s 3p 3d 4s | | | | | | | | v v v v v v v v
Noble Gas Configuration
Elements tend to achieve a stable electron configuration resembling that of noble gases, which have a full outermost shell.
Manganese’s electron configuration is one electron short of the stable configuration of argon (1s 22s 22p 63s 23p 6).
Common Queries: Draw The Electron Configuration For A Neutral Atom Of Manganese.
What is electron configuration?
Electron configuration refers to the distribution of electrons in different energy levels and orbitals around the nucleus of an atom.
How do I determine the electron configuration of a neutral atom?
To determine the electron configuration, follow the Aufbau principle and Hund’s rule, which dictate the order of filling electrons into orbitals based on their energy and spin.
What is the significance of the noble gas configuration?
Atoms tend to achieve a stable noble gas configuration by gaining or losing electrons to attain a full outermost energy level, influencing their chemical reactivity.