Unveiling the enigmatic realm of matter, the Law of Conservation of Matter Worksheet embarks on an enthralling journey to unravel the fundamental principle that governs the unyielding nature of matter. This interactive guide delves into the depths of chemical reactions, historical milestones, and practical applications, illuminating the unwavering truth that matter can neither be created nor destroyed.
From the meticulous experiments of Antoine Lavoisier to the intricate intricacies of nuclear reactions, this worksheet unravels the tapestry of matter’s enduring existence. Engage in hands-on activities, explore real-world examples, and unravel the mysteries that have captivated scientists for centuries.
Law of Conservation of Matter Overview

The law of conservation of matter states that matter can neither be created nor destroyed in a chemical reaction. This means that the total mass of the reactants in a chemical reaction will be equal to the total mass of the products.
This law is one of the fundamental principles of chemistry and has been supported by numerous experiments.
One of the most famous experiments that demonstrates the law of conservation of matter was conducted by Antoine Lavoisier in the 18th century. Lavoisier weighed a sealed glass jar and then added a weighed amount of water to the jar.
He then sealed the jar and heated it until the water boiled. After the water had boiled away, Lavoisier weighed the jar again and found that it had the same mass as it did before he added the water.
This experiment showed that the mass of the water had not been destroyed when it boiled away. Instead, the water had turned into steam, which is a gas. The steam had escaped from the jar, but the total mass of the system (the jar, the water, and the steam) had remained the same.
Applications of the Law of Conservation of Matter: Law Of Conservation Of Matter Worksheet
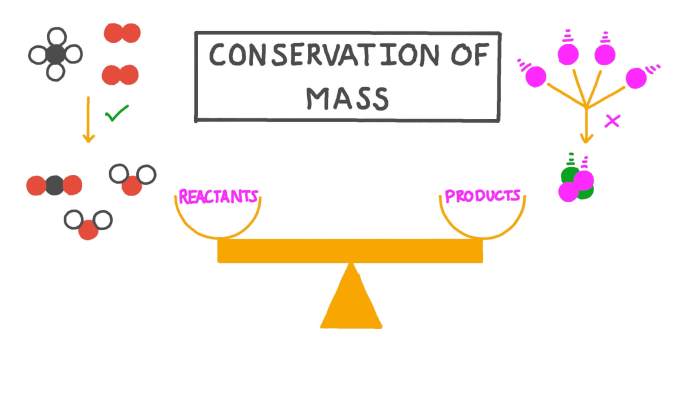
The law of conservation of matter is used in a variety of fields of science, including chemistry, physics, and environmental science.
- In chemistry, the law of conservation of matter is used to balance chemical equations. A balanced chemical equation shows the number of atoms of each element that are present on the reactants’ side of the equation and the number of atoms of each element that are present on the products’ side of the equation.
The law of conservation of matter requires that the total number of atoms of each element be the same on both sides of the equation.
- In physics, the law of conservation of matter is used to explain the conservation of energy. The law of conservation of energy states that energy cannot be created or destroyed. This means that the total amount of energy in a closed system is constant.
The law of conservation of matter is a special case of the law of conservation of energy.
- In environmental science, the law of conservation of matter is used to track the movement of pollutants through the environment. Pollutants can be created or destroyed, but the total amount of pollutants in the environment remains constant. The law of conservation of matter can be used to track the movement of pollutants and to develop strategies to reduce their impact on the environment.
Law of Conservation of Matter Experiments

There are a number of experiments that can be used to demonstrate the law of conservation of matter. One simple experiment is to weigh a piece of paper before and after it is burned. The mass of the paper will be the same before and after it is burned, even though the paper has changed its form.
Another experiment that can be used to demonstrate the law of conservation of matter is to mix two chemicals together and then measure the mass of the products. The mass of the products will be the same as the mass of the reactants, even though the chemicals have changed their form.
Law of Conservation of Matter in Chemical Reactions
The law of conservation of matter applies to all chemical reactions. This means that the total mass of the reactants in a chemical reaction will be equal to the total mass of the products. This law can be used to balance chemical equations and to predict the products of a chemical reaction.
For example, the following chemical equation is balanced:
2H2 + O2 -> 2H2O
This equation shows that two molecules of hydrogen gas (H2) react with one molecule of oxygen gas (O2) to produce two molecules of water (H2O). The total mass of the reactants (2H2 + O2) is equal to the total mass of the products (2H2O).
This is because the law of conservation of matter requires that the total mass of the system remains constant.
Law of Conservation of Matter in Nuclear Reactions
The law of conservation of matter also applies to nuclear reactions. This means that the total mass of the reactants in a nuclear reaction will be equal to the total mass of the products. However, the law of conservation of mass does not apply to the individual atoms that are involved in a nuclear reaction.
This is because nuclear reactions can involve the creation or destruction of atoms.
For example, the following nuclear reaction shows the creation of a new atom:
1H + 2H -> 3He
In this reaction, one atom of hydrogen (1H) and two atoms of deuterium (2H) react to produce one atom of helium (3He). The total mass of the reactants (1H + 2H) is equal to the total mass of the product (3He).
However, the law of conservation of mass does not apply to the individual atoms that are involved in the reaction. This is because the reaction involves the creation of a new atom of helium.
General Inquiries
What is the fundamental principle of the Law of Conservation of Matter?
The Law of Conservation of Matter states that the total mass of matter in an isolated system remains constant, regardless of changes in state or composition.
How did Antoine Lavoisier contribute to the development of the Law of Conservation of Matter?
Lavoisier’s meticulous experiments, particularly his study of combustion, provided crucial evidence supporting the Law of Conservation of Matter.
What are some real-world applications of the Law of Conservation of Matter?
The Law of Conservation of Matter finds applications in fields such as chemistry, environmental science, and nuclear physics, aiding in calculations involving chemical reactions, pollution monitoring, and energy production.